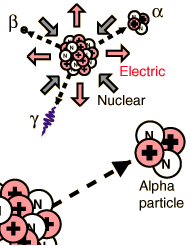
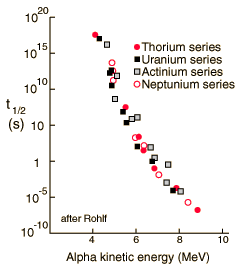
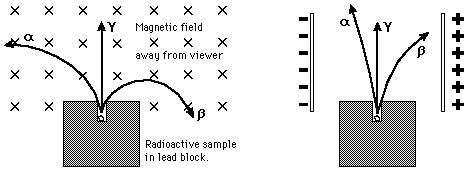
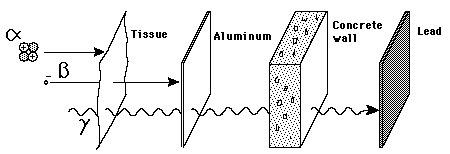
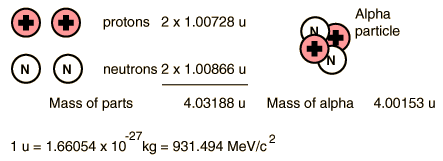| The energy of emitted alpha particles was a mystery to early investigators because it was evident that they did not have enough energy, according to classical physics, to escape the nucleus. Once an approximate size of the nucleus was obtained by Rutherford scattering, one could calculate the height of the Coulomb barrier at the radius of the nucleus. It was evident that this energy was several times higher than the observed alpha particle energies. There was also an incredible range of half lives for the alpha particle which could not be explained by anything in classical physics.
The resolution of this dilemma came with the realization that there was a finite probability that the alpha particle could penetrate the wall by quantum mechanical tunneling. Using tunneling, Gamow was able to calculate a dependence for the half-life as a function of alpha particle energy which was in agreement with experimental observations. |