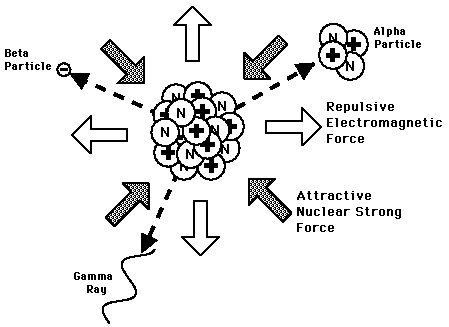
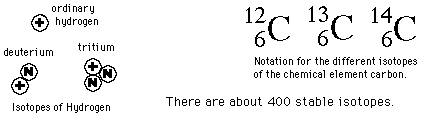Radioactive Dating
Because the radioactive half-life of a given radioisotope is not affected by temperature, physical or chemical state, or any other influence of the environment outside the nucleus save direct particle interactions with the nucleus, then radioactive samples continue to decay at a predictable rate.
If determinations or reasonable estimates of the original composition of a radioactive sample can be made, then the amounts of the radioisotopes present can provide a measurement of the time elapsed.
One such method is called carbon dating, which is limited to the dating of organic (once living) materials. The longer-lived radioisotopes in minerals provide evidence of long time scales in geological processes. While original compositions cannot be determined with certainty, various combination measurements provide self-consistent values for the the times of formations of certain geologic deposits. These clocks-in-the-rocks methods provide data for modeling the formation of the Earth and solar system.
|