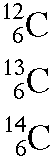Isotopes
The different isotopes of a given element have the same atomic number but different mass numbers since they have different numbers of neutrons. The chemical properties of the different isotopes of an element are identical, but they will often have great differences in nuclear stability. For stable isotopes light elements, the number of neutrons will be almost equal to the number of protons, but a growing neutron excess is characteristic of stable heavy elements. The element tin (Sn) has the most stable isotopes with 10, the average being about 2.6 stable isotopes per element.
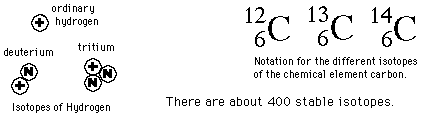
Information about the isotopes of each element and their abundances can be found by going to the periodic table and choosing an element. Then take the link to nuclear data.
|