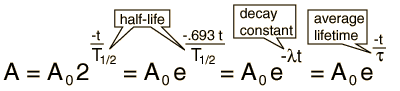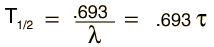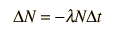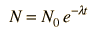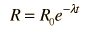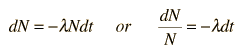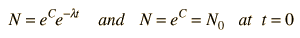Radioactive Half-Life
The radioactive half-life for a given radioisotope is a measure of the tendency of the nucleus to "decay" or "disintegrate" and as such is based purely upon that probability. The tiny nuclear size compared to the atom and the enormity of the forces which act within it make it almost totally impervious to the outside world. The half-life is independent of the physical state (solid, liquid, gas), temperature, pressure, the chemical compound in which the nucleus finds itself, and essentially any other outside influence. It is independent of the chemistry of the atomic surface, and independent of the ordinary physical factors of the outside world. The only thing which can alter the half-life is direct nuclear interaction with a particle from outside, e.g., a high energy collision in an accelerator.
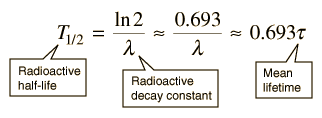
The predictions of decay can be stated in terms of the half-life , the decay constant, or the average lifetime. The relationship between these quantities is as follows.
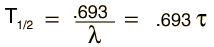
|
Index |