Radiation and the Human Body
Interaction of radiation with matter
Electromagnetic spectrum
| HyperPhysics***** Quantum Physics | R Nave |
Radiation and the Human Body Interaction of radiation with matterElectromagnetic spectrum |
Index | ||
|
Go Back |
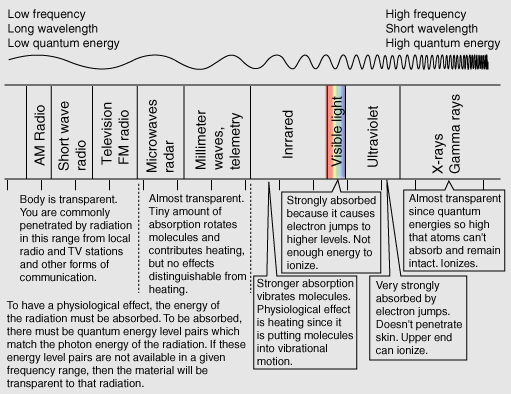
TransparencyYou can see for many miles through clear air and a clear piece of glass obviously is transparent to the wavelengths of visible light. The air is fortunately not transparent to the ultraviolet rays from the sun, though increasing transparency from ozone depletion is a concern. The clear piece of glass is transparent to visible light because the available electrons in the material which could absorb the visible photons have no available energy levels above them in the range of the quantum energies of visible photons. The glass atoms do have vibrational energy modes which can absorb infrared photons, so the glass is not transparent in the infrared. This leads to the greenhouse effect. The quantum energies of the incident photons must match available energy level gaps to be absorbed.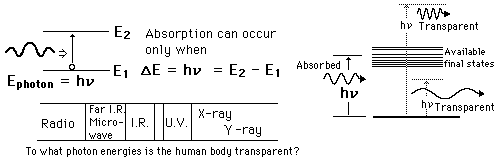 Interaction of radiation with matter |
Index | ||
|
Go Back |
Ionizing Radiation
The mechanisms of interaction for ionizing radiation in the form of x-rays and gamma-rays include the photoelectric effect, Compton scattering and at high enough energies, electron positron pair production. Although the precise ionization energy differs with the atom or molecule involved, a general statement is any radiation with quantum energy above a few electron volts is considered to be ionizing radiation. The threshold for ionization lies somewhere in the ultraviolet region of the electromagnetic spectrum, so all x-rays and gamma-rays are ionizing radiation. All forms of nuclear radiation are also ionizing radiation because of their extremely high energies.
|
Index | |||||
|
Go Back |
Non-Ionizing RadiationIonization is the ejection of one or more electrons from an atom or molecule to produce a fragment with a net positive charge (positive ion). In the electromagnetic spectrum, radiation in the visible or longer wavelength range does not have sufficient quantum energy to ionize an atom, so we classify it as non-ionizing radiation. The threshold for ionization occurs somewhere in the ultraviolet range, with the specific threshold depending upon the type of atom or molecule. It typically takes a photon with energy in the range of a few electron volts to ionize an atom. 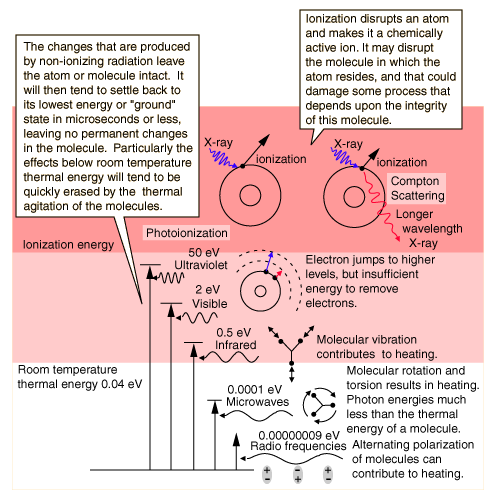 If an atom absorbs a photon of electromagnetic radiation and remains intact, there is a strong tendency for it to return to its ground state. Just as water runs downhill, all physical systems will tend to move to lower energy levels. If the quantum energy of the radiation absorbed is higher than the average thermal energy of the molecules (that is, infrared or visible radiation), then the downward transitions may emit radiation that leaves the material, or it may be gradually transformed into general thermal energy in the material. Radiation in the microwave or longer wavelengths generally just contributes to the random molecular motion which we have described as thermal energy. The net result of the absorption of non-ionizing radiation is generally just to heat the sample. Of course if it heats it enough, then chemical changes are likely to occur, but those chemical changes would be expected to be the same changes that would occur as a result of any other source of heating.
|
Index | ||
|
Go Back |