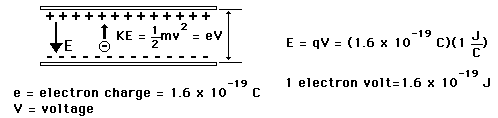Energies in Electron Volts
Room temperature thermal energy of a molecule..................................0.04 eV
Visible light photons....................................................................................1.5-3.5 eV
Energy for the dissociation of an NaCl molecule into
Na+ and Cl- ions:.............................................................................................4.2 eV
Ionization energy of atomic hydrogen ........................................................13.6 eV
Approximate energy of an electron striking a color
television screen...................................................................................20,000 eV
High energy diagnostic medical x-ray photons...............................200,000 eV
(=0.2 MeV)
Typical energies from nuclear decay:
(1) gamma..................................................................................................0-3 MeV
(2) beta.......................................................................................................0-3 MeV
(3) alpha....................................................................................................2-10 MeV
Cosmic ray energies ........................................................................1 MeV - 1000 TeV
|