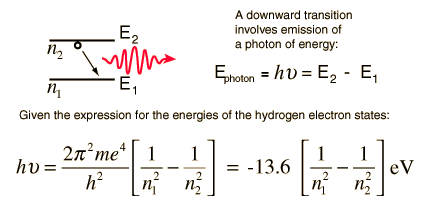
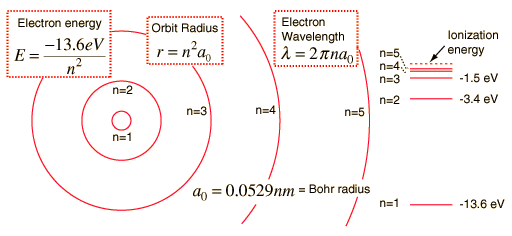
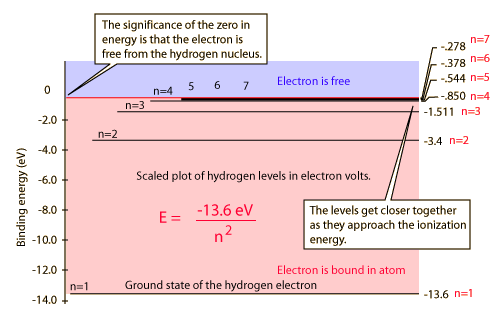
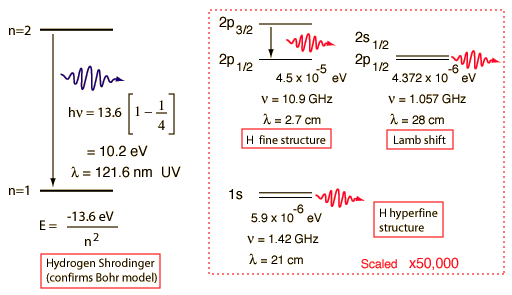
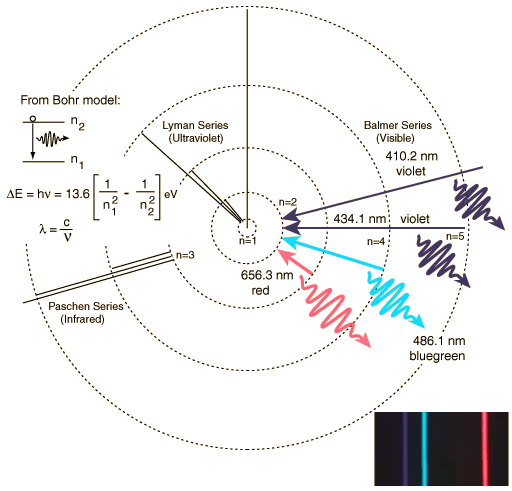
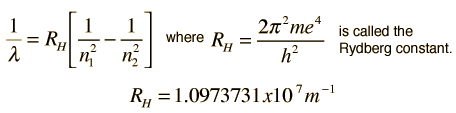Hydrogen Spectrum
This spectrum was produced by exciting a glass tube of hydrogen gas with about 5000 volts from a transformer. It was viewed through a diffraction grating with 600 lines/mm. The colors cannot be expected to be accurate because of differences in display devices.
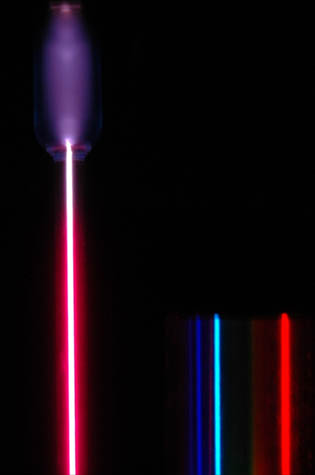 |
At left is a hydrogen spectral tube excited by a 5000 volt transformer. The three prominent hydrogen lines are shown at the right of the image through a 600 lines/mm diffraction grating.
An approximate classification of spectral colors:
- Violet (380-435nm)
- Blue(435-500 nm)
- Cyan (500-520 nm)
- Green (520-565 nm)
- Yellow (565- 590
nm)
- Orange (590-625 nm)
- Red (625-740 nm)
|
Radiation of all the types in the electromagnetic spectrum can come
from the atoms of different elements. A rough classification of some of
the types of radiation by wavelength is:
- Infrared > 750 nm
- Visible 400 - 750 nm
- Ultraviolet 10-400 nm
- Xrays < 10 nm
|