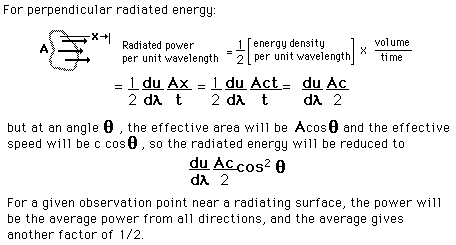
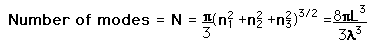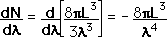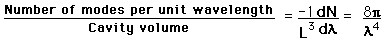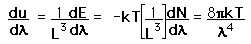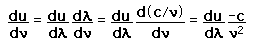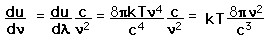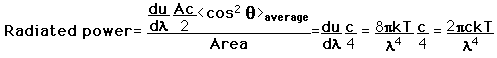How many modes in the cavity?
From the standing wave solution to the wave equation we get the condition
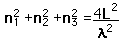 . .
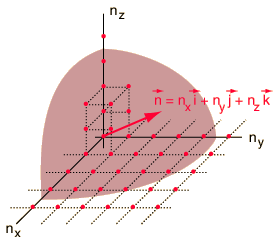
The Rayleigh scheme for counting modes.
After Richtmyer, et al.
| We need to evaluate the number of modes which can meet this condition, which amounts to counting all the possible combinations of the integer n values. An approximation can be made by treating the number of combinations as the volume of a three-dimensional grid of the values of n, an "n-space". Using the relationship for the volume of a sphere, with the n values specifying the coordinates along three "n" axes, gives
|
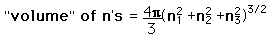 . .
|
This has a couple of problems, however. In using a sphere, we have used both positive and negative values of n, whereas the wave equation solution uses only positive definite values. Therefore we must take 1/8 th of the volume above. Another technical problem is that you can have waves polarized in two perpendicular planes, so we must multiply by two to account for that. Then the volume can be taken to be a measure of the number of modes, becoming a very good approximation when the size of the cavity is much greater than the wavelength as in the case of electromagnetic waves in finite cavity. Using the relation obtained for the values of n, this becomes
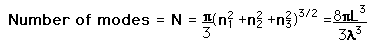
|
Index
Rayleigh- Jeans references
Richtmyer, et al.
Ch. 5
Blackbody radiation concepts |
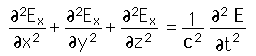
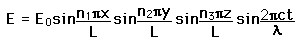
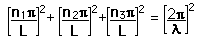
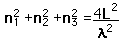

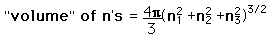 .
.