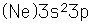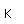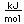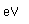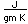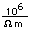Aluminum
Aluminum is an abundant, light, and strong metal which has found many uses. The main drawback to its use is the large amount of energy necessary to refine it from its common ore, bauxite. The recycling of aluminum scrap metal saves over 90% of the energy required to separate aluminum from bauxite.
Aluminum is only about one third as dense as iron, but some of its alloys, such as duraluminum are as strong as mild steel. Duraluminum is formed from 94.3% aluminum, 4% copper, 0.5% manganese, 0.5% magnesium, and 0.7% silicon. While much stronger than pure aluminum, this alloy is less resistant to corrosion and is often clad with pure aluminum.
Because of its lightness and strength, aluminum is used widely in aircraft construction. It has high electrical conductivity, 80% of that of copper, and is used in place of copper in large electrical conductors. Its drawback there is its tendency to oxidize at contacts, requiring the use of contacts coated with an antioxidant.
Aluminum is a very active metal, a fact often disguised by the fact that it rapidly forms barrier oxide layers on exposed surfaces, inhibiting its interaction. When strongly heated it burns rapidly in air, and in the form of a fine dust is explosive.
Aluminum is one of the big 8 elements in the Earth's crust, being the third most abundant element at about 8.1% by weight. It is a constituent of the class of silicates called feldspars, which are the most abundant minerals in the Earth's crust. It is also a constituent of mica. One aluminum silicate mineral is pyrophyllite, AlSi2O5(OH). Aluminum combines with other metals in many silicates, e.g., manganese in spessartine, Mn3Al2(SiO4)3. One aluminum silicate, Al2SiO4 (F,OH)2, forms the gem mineral topaz. A silicate with potassium , KAlSi3O8, forms the mineral microcline, which can be apple green to brown in color. A silicate with calcium, zoisite forms blue gems.
Aluminum oxide, Al2O3, is called alumina and occurs in nature as the mineral corundum. Corundum and impure corundum called emery are used in making abrasive cloths and wheels. Pure corundum is colorless, but the addition of a small impurity of chromic oxide can make the precious stone ruby (red) and the addition of titanium oxide can make sapphire (blue and other colors).
Aluminum along with magnesium form the oxide mineral spinel. One aluminum phosphate mineral is variscite, AlPO4.2H2O, which exhibits an apple green color and is popular as an ornamental stone.
Aluminum sulfate is used in water purification. It is also used as a mordant in the dying of cloth. A mordant fixes the dye to the cloth, rendering it insoluble.
Aluminum has excellent reflective properties and is widely used in evaporated coatings for the manufacture of mirrors.
|
Index
Periodic Table
Chemistry concepts
Reference
Pauling
Ch. 26
Schad
Ch. 15 |