Liquid Helium Working Range
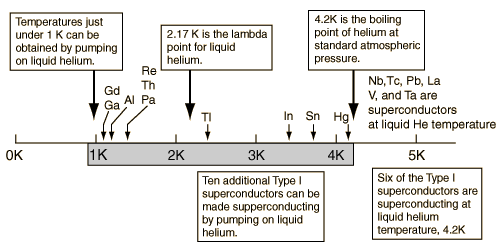
Liquid helium | Lambda point | Superconductors |
Reference:
Blatt
| HyperPhysics***** Quantum Physics | R Nave |
Liquid HeliumKamerlingh Onnes worked for many years to liquify the element which persisted as a gas to the lowest temperature. Using liquid air to produce liquid hydrogen and then the hydrogen to jacket the liquification apparatus, he produced about 60 cubic centimeters of liquid helium on July 10, 1908. Its boiling point was found to be 4.2 K. Onnes received the Nobel Prize in 1913 for his low temperature work leading to this achievement. When helium is cooled to a critical temperature of 2.17 K (called its lambda point), a remarkable discontinuity in heat capacity occurs, the liquid density drops, and a fraction of the liquid becomes a zero viscosity "superfluid". Superfluidity arises from the fraction of helium atoms which has condensed to the lowest possible energy. An important application of liquid helium has been in the study of superconductivity and for the applications of superconducting magnets. Liquid helium working range |
Index | ||
|
Go Back |
Liquid Helium Working Range
|
Index Reference: Blatt | |||
|
Go Back |
SuperfluidityA remarkable transition occurs in the properties of liquid helium at the temperature 2.17 K, called the "lambda point" for helium. Part of the liquid becomes a "superfluid", a zero viscosity fluid which will move rapidly through any pore in the apparatus. A vacuum container which seemed to be leak tight could suddenly leak helium rapidly as the superfluid moved out through a microscopic hole. A vertical tube could produce a fountain effect as the superfluid moved up the walls and out the top. In 1938, F. London proposed a "two-fluid" model to explain the behavior of the liquid: normal liquid and the superfluid fraction consisting of those atoms which have "condensed" to the ground state and make no contribution to the entropy or heat capacity of the liquid. This condensed fraction is the standard example of Bose-Einstein condensation. Another remarkable characteristic of the the superfluid is its very high heat conductivity, 30 times that of copper! Application in IRAS Satellite |
Index Reference: Blatt | ||
|
Go Back |
Lambda Point for Liquid HeliumWhen helium is cooled to a critical temperature of 2.17 K , a remarkable discontinuity in heat capacity occurs, the liquid density drops, and a fraction of the liquid becomes a zero viscosity "superfluid". It is called the lambda point because the shape of the specific heat curve is like that Greek letter. Superfluidity arises from the fraction of helium atoms which has condensed to the lowest possible energy by a process called Bose-Einstein condensation.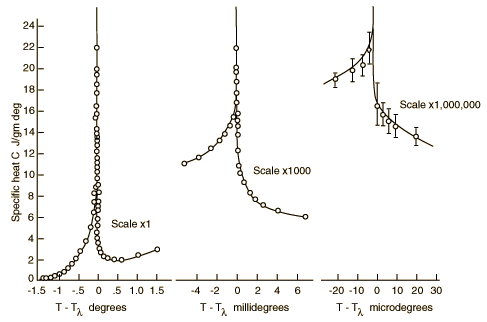 Specific heat data from Buckingham and Fairbank, 1961, p. 138. Liquid helium working range |
Index Reference: Blatt | ||
|
Go Back |