Acid-Base Reactions
When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt. The H(+) cation of the acid combines with the OH(-) anion of the base to form water. The compound formed by the cation of the base and the anion of the acid is called a salt. The combination of hydrochloric acid and sodium hydroxide produces common table salt, NaCl:
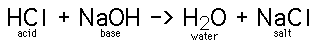
The word salt is a general term which applies to the products of all such acid-base reactions.
| Inorganic Compounds |
Periodic Table
Chemistry concepts
Chemical reaction concepts
| HyperPhysics | R Nave |