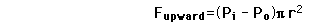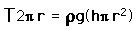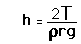Surface Tension and Bubbles
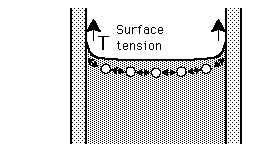
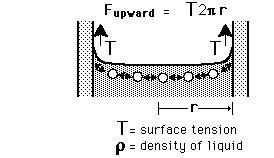
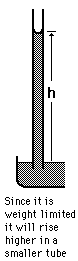
The surface tension of water provides the necessary wall tension for the formation of bubbles with water. The tendency to minimize that wall tension pulls the bubbles into spherical shapes (LaPlace's law).
The pressure difference between the inside and outside of a bubble depends upon the surface tension and the radius of the bubble. The relationship can be obtained by visualizing the bubble as two hemispheres and noting that the internal pressure which tends to push the hemispheres apart is counteracted by the surface tension acting around the cirumference of the circle.
For a bubble with two surfaces providing tension tension, the pressure relationship is:
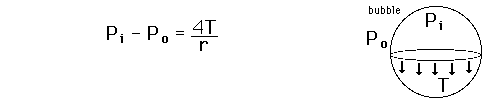
Derive the relationship
Fluid concepts
| HyperPhysics***** Mechanics ***** Fluids | R Nave |