The Electric Pickle
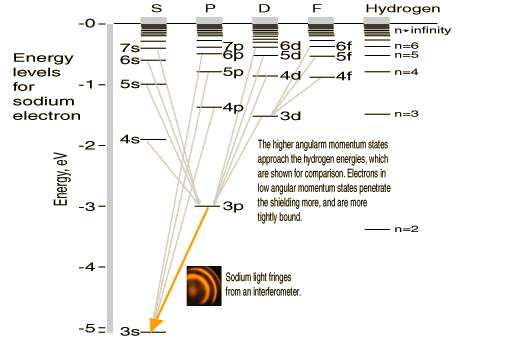
A far-fetched example of a non-ohmic resistor is the electric pickle. A considerable amount of light can be obtained by connecting ordinary household 120 volt AC voltage across a pickle. After the pickling process, there are Na+ and Cl- ions present. The standard explanation is that the electric current excites the sodium ions, producing light similar to
that of a sodium lamp.

Thanks to Brian Lucy for this example. | Currents in ionic solutions are often not linearly proportional to the applied voltage. When Ohm's law is used with ordinary carbon resistors, the ratio of voltage to current is constant, but the variation in light output suggests that this is not the case with the electric pickle. |
|
Index |