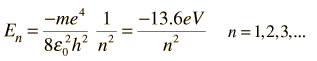Radial Equation Separation
Substituting the form
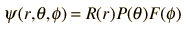
into the hydrogen Schrodinger equation allows all the partial derivatives to be expressed as ordinary derivatives. Direct substitution and a bit of rearranging gives
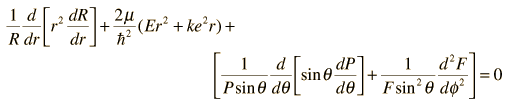
Note that the first two terms contain only variable r, and the remaining terms contain the angular variables. Since the equation must hold for all values of these variables, the collection of radial terms must be equal to a constant. This separates out the radial equation. The sum of the angular terms must therefore be equal to the negative of that constant. Also, the terms in the two angles must separately equal a constant since they must hold for all angle values. This separates the two angular parts into the colatitude and azimuthal equations.
Schrodinger equation concepts
Hydrogen concepts
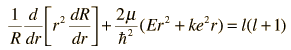
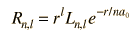
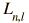 is the associated Laguerre function. The first few radial wavefunctions R are shown as part of the
is the associated Laguerre function. The first few radial wavefunctions R are shown as part of the