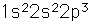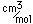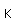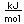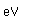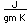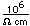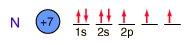Nitrogen
Nitrogen is a colorless, odorless, and tasteless gas which is composed of diatomic molecules N2. It constitutes 78% of the air by volume. At 0°C and 1 atmosphere pressure, a liter has a mass of 1.2506 grams. The gas condenses to a colorless liquid at 77.25 K and to a white solid at 63.3 K.
Nitrogen is chemically unreactive. It does not burn, and at ordinary temperatures it does not react with other elements. It is prepared commercially by distillation of liquid air. Liquid nitrogen is extremely useful in the laboratory for cooling and pumping applications.
Nitrogen is an essential element in most of the substances which make up living organisms, including the proteins. Important compounds include fertilizers and explosives.
|