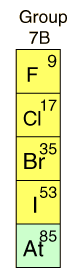
 | The HalogensThe elements of Group VII of the Periodic Table are called halogens, which means "salt formers". They lack only one electron to form a complete shell or subshell, and are extremely active chemically. Chemical activity increases as you move upward in the group, fluorine being the most active element in the Periodic Table. At room temperature, fluorine and chlorine are gases, bromine is liquid, and iodine is solid. They form diatomic molecules in the pure state. The halogens are poisonous, and chlorine gas and chlorine compounds have been used as chemical weapons. In small concentrations, chlorine is used to disinfect drinking water and to disinfect swimming pools. |
| Chemistry of the Elements |
Periodic Table
Chemistry concepts
| HyperPhysics | R Nave |