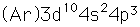Arsenic
Ordinary gray arsenic is a semi-metallic substance, steel-gray in color with density 5.73. There is also an unstable yellow crystalline form containing As4 molecules, similar to the structure of phosphorus.
In nature arsenic is found in several ores. Orpiment, As2S3, is a yellow substance while realgar, AsS, is a red substance. Stibarsen, SbAs, is a silvery mineral with a metallic luster. It is also found in the oxide arsenolite, As4O6, and in arsenopyrite, FeAsS. A sulfide with copper is called enargite. Dufrenoysite and gratonite are sulfides of arsenic with lead. A sulfide formed with lead and antimony is called jordanite. Arsenic forms a sulfide mineral with nickel called gersdorffite. Tennantite is a sulfide which contains arsenic, copper, iron and antimony. Arsenic joins with iron and cobalt in the sulfide glaucodot. In the sulfide mineral hutchinsonite, (Tl, Pb)2As5S9, arsenic joins with thallium and lead. Arsenic forms a compound with platinum, PtAs2 which appears in the mineral sperrylite. Cobalt and arsenic form the mineral safflorite, CoAs2. Arsenates with copper and zinc such as bayldonite, adamite and olivenite have a deep green color. Ludlockite is an arsenate with iron and lead and has a coppery red color. Mimetite, an arsenate of lead with chlorine, forms yellow crystals. Scorodite is an arsenate of iron and reinerite is an arsenate of zinc.
Arsenic is used with lead (0.5% As) in making lead shot. It makes the metal harder than the pure lead and improves the properties of the molten material. The shot is formed by pouring the molten material through a screen at the top of a tower and allowing it to solidify into spherical balls before reaching water in a collecting vessel at the bottom of the tower.
Arsine, AsH3, is a very poisonous gas with a garlic-like odor. Arsenic metal is very toxic, as are many of its compounds. Cupric hydrogen arsenate and a cupric arsenic-acetate (called Paris green) are used as insecticides. Sodium arsenate, Na3AsO4,is used as a weed killer, and calcium arsenate is used as an insecticide. The toxicity of arsenic compounds is sometimes used to advantage in medicine by devising chemotherapy where arsenic compounds at lower than poisonous doses to man are used to attack invading microorganisms.

|
Arsenic is one of the few elements which can be found in nature in pure form. This sample of arsenic is displayed in the Smithsonian Museum of Natural History. The sample is about 18x18 cm and is from Andreasberg, Niedersachsen, Germany.
|
|
Index
Periodic Table
Chemistry concepts
Reference
Pauling
Ch. 16 |