The Size of Atoms
The size of atoms can be estimated with the use of Avogadro's number along with the atomic mass and bulk density of a solid material. From these, the volume per atom can be determined.
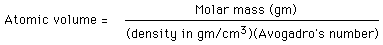
The cube root of the volume is an estimate of the diameter of the atom. For carbon, the molar mass is exactly 12, and the density is about 2 gm/cm^3. The estimated volume is then
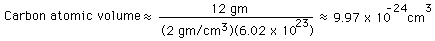
and the estimate of the carbon atomic diameter is the cube root of that.
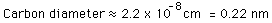
This estimate is a bit small. It can be refined somewhat by considering the atoms to be spheres and packing them in different ways. Carbon in diamond form has a different density than graphite because of its atomic lattice structure. But this estimate at least establishes the kind of atomic sizes expected. A typical atomic diameter is 0.3 nm.
| Graph of atomic radii |
| How do you find out about things you can't see? |
Particle concepts
Why higher energy?
Survey of scattering
Scattering concepts