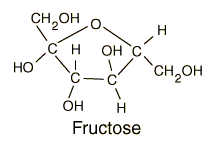
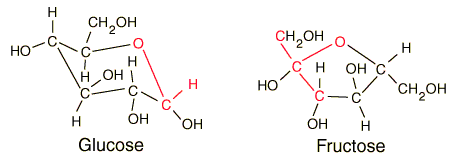
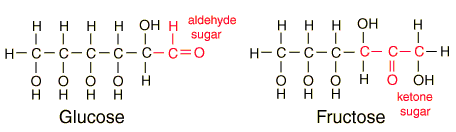
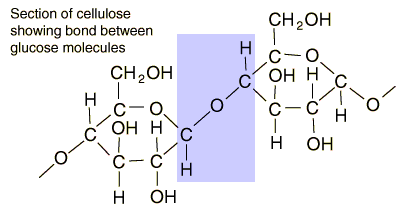
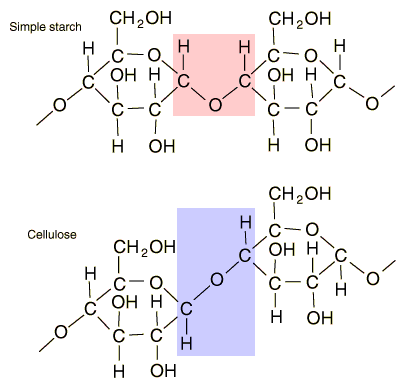
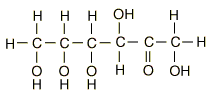Glucose
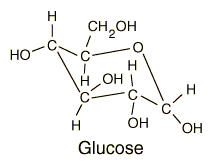 |
Glucose is a carbohydrate, and is the most important simple sugar in human metabolism. Glucose is called a simple sugar or a monosaccharide because it is one of the smallest units which has the characteristics of this class of carbohydrates. Glucose is also sometimes called dextrose. Corn syrup is primarily glucose. Glucose is one of the primary molecules which serve as energy sources for plants and animals. It is found in the sap of plants, and is found in the human bloodstream where it is referred to as "blood sugar". The normal concentration of glucose in the blood is about 0.1%, but it becomes much higher in persons suffering from diabetes. |
When oxidized in the body in the process called metabolism, glucose produces carbon dioxide, water, and some nitrogen compounds and in the process provides energy which can be used by the cells. The energy yield is about 686 kilocalories (2870 kilojoules) per mole which can be used to do work or help keep the body warm. This energy figure is the change in Gibbs free energy DG in the reaction, the measure of the maximum amount of work obtainable from the reaction. As a primary energy source in the body, it requires no digestion and is often provided intravenously to persons in hospitals as a nutrient.
Energy from glucose is obtained from the oxidation reaction
where a mole of glucose (about 180 grams) reacts with six moles of O2 with an energy yield DG = 2870 kJ. The six moles of oxygen at STP would occupy 6 x 22.4L = 134 liters. The energy yield from glucose is often stated as the yield per liter of oxygen, which would be 5.1 kcal per liter or 21.4 kJ per liter. This energy yield could be measured by actually burning the glucose and measuring the energy liberated in a calorimeter. But in living organisms, the oxidation of glucose contributes to a series of complex biochemical reactions which provides the energy needed by cells. In animal cells these processes have been modeled in the Krebs cycle. A major part of the use of the energy from glucose oxidation is the conversion of ADP to ATP, with the energy-rich molecule ATP being subsequently used as the energy currency of the cell.
Glucose is manufactured by plants with the aid of energy from the sun in the process called photosynthesis. This synthesis is carried out in the small energy factories called chloroplasts in plant leaves. The chloroplasts capture the energy from light and fabricate glucose molecules from carbon dioxide from the air and water from the soil.
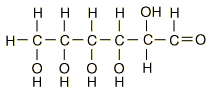 | Glucose can also be found in a linear form. The right end of this molecule shows the form of an aldehyde. |
| Comparison of glucose and fructose |
Biochemical concepts
Chemistry concepts
References
Shipman, Wilson and Todd
Ch 15
Tillery, Enger and Ross
Ch 14
Tuszynski and Dixon
Ch 15
| HyperPhysics*****Chemistry | R Nave |