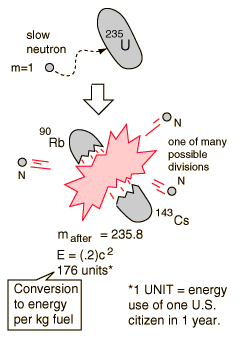
Nuclear Fission
If a massive nucleus like uranium-235 breaks apart (fissions), then there will be a net yield of energy because the sum of the masses of the fragments will be less than the mass of the uranium nucleus. If the mass of the fragments is equal to or greater than that of iron at the peak of the binding energy curve, then the nuclear particles will be more tightly bound than they were in the uranium nucleus, and that decrease in mass comes off in the form of energy according to the Einstein equation. For elements lighter than iron, fusion will yield energy.
The fission of U-235 in reactors is triggered by the absorption of a low energy neutron, often termed a "slow neutron" or a "thermal neutron". Other fissionable isotopes which can be induced to fission by slow neutrons are plutonium-239, uranium-233, and thorium-232.
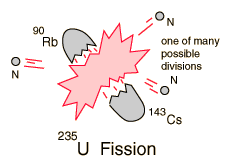
| Uranium-235 Fission |
Fission concepts
| HyperPhysics***** Nuclear | R Nave |