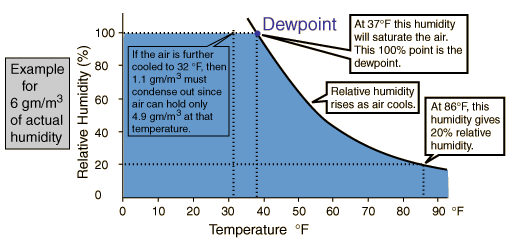
Relative Humidity
The amount of water vapor in the air at any given time is usually less than that required to saturate the air. The relative humidity is the percent of saturation humidity, generally calculated in relation to saturated vapor density.

The most common units for vapor density are gm/ . For example, if the actual vapor density is 10 gm/m3 at 20°C compared to the saturation vapor density at that temperature of 17.3 gm/m3 , then the relative humidity is
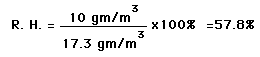 | Calculation |
| Saturation vapor pressure | Dewpoint | Relative humidity calculation |
Kinetic theory concepts
Applications of kinetic theory
Vapor application concepts
| HyperPhysics***** Thermodynamics | R Nave |