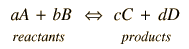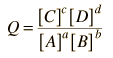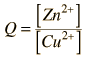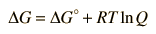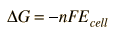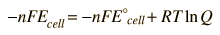Calculation of Voltaic Cell Potentials
When an electrochemical cell is arranged with the two half-reactions separated but connected by an electrically conducting path, a voltaic cell is created. The maximum voltage which can be produced between the poles of the cell is determined by the standard electrode potentials under the standard conditions under which those potentials are defined.
Consider the historic Daniell cell in which zinc and copper were used as electrodes. The data from the table of standard electrode potentials is
| Cathode (Reduction) Half-Reaction | Standard Potential E° (volts) |
| Zn2+(aq) + 2e- -> Zn(s) | |
| Cu2+(aq) + 2e- -> Cu(s) |
The cell potential can be written
Ecell = oxidation potential + reduction potential
Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get its oxidation potential. When that is done, it is clear that the theoretical standard cell potential for the zinc-copper cell is 1.10 volts.
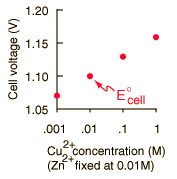
In general, a real voltaic cell will differ from the standard conditions, so we need to be able to adjust the calculated cell potential to account for the differences. This can be done with the application of the Nernst equation.
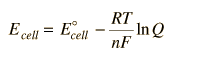
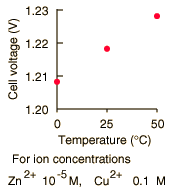
By numerically exploring the above calculation, you can confirm that large concentration differences between the anions and the cations can cause the voltage to differ significantly from the standard cell potential, and that such large concentration differences also make the cell potential more temperature dependent.
Oxidation/
Reduction concepts
Electrochemistry concepts
Reference
Hill & Kolb
Ch 8
Ebbing
Ch 19
| HyperPhysics***** Electricity and Magnetism ***** Chemistry | R Nave |